February - March 2004: Ipsilateral Suppression of Transiently Evoked Emissions
- Details
- Category: Guest editorial
- Last Updated on Wednesday, 02 April 2014 14:22
- Written by George A. Tavartkiladze, PhD
- Hits: 5675
This Editorial is a Synopsis from the chapter on Ipsilateral Suppression by George A. Tavartkiladze, Gregory I. Frolenkov, Alexandr V. Kruglov, Serge V. Artamasov in the book Otoacoustic Emissions and Clinical Applications . M. Robinette & T. Glattke editors,
1. Introduction
Many studies have been devoted to the suppression of transient evoked otoacoustic emissions (TEOAEs) by contralateral acoustic stimulation starting with the 1993 paper by the Collet group in France. It is believed that this effect is mediated by the medial olivocochlear system (Durrant, 1998; Veuillet, Collet & Morgon, 1992), and it is relatively small. Typically the TEOAE suppression associated with contralateral stimuli of 70-75 dB SPL is about 1 to 2 dB (Veuillet et al., 1991). In contrast to contralateral stimulation, ipsilateral masking can result in more pronounced suppression of TEOAE (Kemp & Chum, 1980; Tavartkiladze et al., 1993; Wilson, 1980). The mechanisms underlying this effect seem to be twofold. From one view, the suppression results from intracochlear masking processes; from another view, it appears to be mediated through the olivocochlear system. This chapter describes various aspects of TEOAE masking properties under simultaneous and forward masking conditions that have been investigated for several years (Frolenkov et al., 1995; Tavartkiladze, et al., 1991, 1996).
2. Ipsilateral Simultaneous Masking of TEOAEs
Since the first description by Kemp (1978), the measurements of TEOAEs have progressed from laboratory research to clinical application. Today it is universally accepted that OAE phenomena are of cochlear origin (Probst, 1991; Zurek, 1985). Nevertheless, the particular segments of the cochlear partition generate TEOAE in response to a stimulus with given frequency composition remain unresolved (Hilger et al., 1995; Kemp 1986; Tavartkiladze et al., 1993). This situation is due to the very complex structure of the TEOAE frequency spectrums and to the fact that not all frequencies evoke TEOAEs (Probst et al., 1986). Constructing TEOAE tuning curves under simultaneous tonal masking conditions can reveale information about the location of the cochlear partition vibration maximum. Unfortunately, only a few researchers have described the results of TEOAE simultaneous masking investigations (Kemp & Chum, 1980; Wilson, 1980).
3. Simultaneous-Masking Procedures
All our simultaneous masking experiments were performed with subjects who were 21 to 33 years of age, with audiometric thresholds less than 20 dB HL within the frequency range of 125-8000 Hz, with type A tympanograms, and no signs of otologic diseases during the investigation. Because spontaneous otoacoustic emissions (SOAEs) could modify TEOAE responses (Probst et al., 1986), the existence of SOAE or (synchronized) quasi-SOAEs was tested using an ILO 88 system (Otodynamics, Hatfield, UK).
TEOAEs were recorded with a custom-designed acoustic probe, consisting of a microphone (EA-1842) and two Knowles (Itasca, IL) electroacoustic transducers (ED-1913) (Fig. 1). The free field calibration of the probe microphone was carried out by short broadband clicks with initial rarefaction wave. The probe under calibration was placed close to the measuring microphone (4676, Bruel & Kjaer, Nram, Denmark) connected to a measuring amplifier (2235 noise meter, Bruel & Kjaer). Output of the measuring amplifier was used for calibration of the probe. (The frequency response of the probe-microphone channel is presented in the left bottom panel of Fig. 1).
During the experiments, probe-microphone output was amplified and fed to a Medelec "Sensor-3" clinical averager using an effective filter bandwidth of 300 Hz (6 dB per octave) to 6000 Hz (12 dB per octave). One of the electroacoustical transducers was used to deliver test stimuli, which were 60 and 500 microsecond clicks, and tone bursts of different frequencies with trapezoidal envelope: 1 cycle rise/fall, 1 cycle plateau for frequencies less than 1 kHz, and 2 cycles rise and fall, 3 cycles plateau for frequencies more than 1 kHz. To reduce inter-subject variability of TEOAE amplitude, the stimulus intensity was related to the subject's sensation level and set at 20 dB SL to provide selective excitation of the limited segment of cochlear partition. Test stimuli repetition rate was 20 Hz.
TEOAE-response waveforms were obtained with synchronous averaging of 2000 consecutive responses to test stimuli. The signal was then routed into two independent channels of averaging system. Thus, averaged responses to even and odd stimuli were obtained. The sum of these curves formed the TEOAE record, and the difference between them was used for noise-level estimation. The second electroacoustical transducer was employed to deliver masking tones of different frequencies. First, the subjective threshold of tone perception was determined for each frequency. After that, masking was continued with constant intensity during the averaging process. Any intensity changes were performed at least 1-2 min before the start of averaging. For masker-artifact cancellation, the reference masker was attenuated, phase-corrected, and electrically added to the probe-microphone output (Fig.1) before leading it to the averager. The degree of attenuation and phase correction angle were manually adjusted in such a way as to minimize amplitude of the signal at the averager input. The adjustment was necessary each time when the frequency or intensity of the masker tone were changed.
The results obtained were used for the construction of iso-suppression tuning curves. For each frequency of masking tone, the relation between the masker intensity (5-60 dB SL) and TEOAE amplitude was determined. Then the masking tone intensity necessary for 50% reduction of TEOAE amplitude was approx20uation was less than 50%, even under the highest levels of masking tone (50-60 dB SL), the masking tone of this frequency was not considered to produce TEOAE reduction and this intensity was marked by the arbitrary value of 180 dB.

Fig 1: Schematic drawing of the simultaneous masking experiments setup. Left bottom panel represents the probe-microphone frequency response
4. TEOAE-Amplitude Calculation
Linear component cancellation (Bray & Kemp, 1987; Kemp,et al., 1986) was not suitable for these experiments, because the method dramatically reduced the amplitude of TEOAE evoked by stimuli of a relatively low intensity (Frolenkov et al., 1995; Grandori & Ravazzani, 1993; Tavartkiladze et al., 1994). Instead, TEOAEs were recoeded by ordinary averaging. However, the linear component cancellation was used for the determination of time window of analysis. For this purpose, the control TEOAE recordings were made at different click intensities (0-46 dB SL), and for each subject the difference was obtained between TEOAE records to 30 dB SL click and to 20 dB SL click after multiplying the latter record by a 10 dB correction coefficient (Fig. 2). The difference consisted of non-linear TEOAE components only and was used to determine the analysis window (Fig. 2). Additionally, the appropriateness of the time window estimate was determined by the construction of input/output curves for the RMS amplitude of TEOAE in time intervals shorter than window chosen. Any time intervals that did not include non-linear TEOAE components were excluded from consideration. Hamming window function and Fast Fourier Transform (FFT) was performed. The amplitude of the TEOAE was calculated as the square root of the difference between the signal power and noise power in the frequency range where the spectrum of signal exceeded that of noise more than 3 dB.

Fig.2. - Analysis time determination. A, B: TEOAE responses evoked by 50 s clicks with intensity of 30 dB SL and 20 dB SL correspondingly. C: The difference of the above recordings after multiplying of the record by the 10 dB correction coefficient. This difference consists of non-linear TEOAE components only and was used to determine the analysis window (indicated by horizontal line). The zero point on the time scale corresponds to the stimuli onsets. Stimulus artifact on record C was not completely cancelled due to the signal limitation by the ADC converter.
5. TEOAE Tuning Properties
The TEOAEs were suppressed in all the experiments with simultaneous tonal masking. Fig. 3 shows typical TEOAEs recorded in response to 1.5-kHz tone bursts without masking and with masking tone of various intensities. In the experiments, the masking effect increased directly with the sensation level of the masking tone. With the tone at 40 dB SL, almost total suppression of TEOAE was observed. Such significant response suppression was found only at the masking-tone frequency equal or close to that of the tone burst (Fig. 3).
Fig.3: TEOAE reduction under simultaneous masking by 1.5-kHz tone of increasing intensity (indicated on the records). OAEs were evoked by 1.5-kHz, 20-dB SL tone bursts.
Fig. 4 shows typical iso-suppression tuning curves of tone-burst and click-evoked OAEs. In all subjects and for all stimuli the tuning curve corresponded to the TEOAE frequency spectrum. In the case of tone-burst stimulation, locations of the maxima of the TEOAE and stimulus spectrum and tuning-curve tip were the same. The tuning properties of click-evoked OAE were different, and TEOAE spectral maxima, as well as TEOAE tuning-curves tips, did not correspond to the stimulus spectra. Indeed, clicks of various duration (60 microseconds and 500 microseconds) had quite different spectra . Irrespective of the click-stimuli spectra, the spectra of TEOAE were practically identical, and correspondingly similar TEOAE tuning-curves were obtained. In addition, the smaller difference between the tip and the low-frequency segment of the tuning curve with the 500 microseconds stimulus closely correlated to the existence of low-frequency TEOAE components which were not observed for the 60 microseconds click stimulation. In this subject tone burst stimulation with a frequency of 1.5 kHz was the most effective for the TEOAE excitation . The TEOAE evoked by this stimulus had in its spectrum essentially the same peaks that dominated in the spectra of TEOAE to broad-band stimulation . As a result, the tuning curve of the TEOAE to the 1.5 kHz tone burst was similar to the tuning curves of click-evoked OAEs (Fig.6-6). TEOAE spectra of all test subjects were characterized by the dominant peaks within the 1 to 2 kHz range, and the peaks strictly determined the tuning curves of both OAEs evoked by clicks of different duration and TEOAE to the tone burst of the most effective frequency. Nevertheless, this apparent independence of TEOAE-frequency composition and iso-suppression tuning curves from the stimuli spectra was only relative. When the stimulus energy was concentrated within the frequency range which did not comprise the frequencies of the dominant peaks of TEOAE to broad-band stimulation, the TEOAE with different frequency composition was observed . For example, OAEs evoked by 2.5 kHz tone bursts had the spectral peaks within the range of 1.8 to 2.6 kHz and the tuning curve with the tip located around 2.5 kHz (Fig. 4). Comparison of the tone-evoked OAE tuning curves showed that OAEs evoked with 2.5 kHz tone burst was characterized by a somewhat wider tuning curve than the TEOAEs to 1.5 kHz stimulation. This difference was not surprising considering the wider spectrum of TEOAE to the 2.5 kHz tone burst (Fig. 4). The tuning-curves shape with typical flat low frequency "plateau" and steep high frequency rise (Fig. 4) was observed in all subjects.

Fig. 4. Iso-suppression tuning curves (top) of OAEs evoked by clicks of different durations (A) and by tone bursts of different frequencies (B). Bottom records show from top to bottom: the spectra of stimuli and the spectra of corresponding TEOAEs. Stimuli intensity was 20 dB SL. Dashed lines indicate noise level.
The relation of TEOAE simultaneous-masking properties to the TEOAE-frequency composition was further explored by construction of tuning curves of the separate TEOAE frequency components (Fig. 5). It was found that the components were suppressed independently and had individual tuning curves with the typical shape (Fig. 4). The tips of the tuning curves were closely related to the frequency of separate components (Fig. 5). The intensities of masking tones that corresponded to the tuning curves tips tended to be higher for dominant peaks (Fig. 5). Usually the amplitudes of the TEOAE frequency components differed, and the tuning curve constructed from total TEOAE spectrum was determined by the contribution of TEOAE dominant spectral peaks (Fig. 5). Finally, the the independent suppression of the TEOAE frequency components was observed in all the subjects. Unfortunately, neither subjects had SOAEs, and it could not be determined how the presence of SOAEs could modify the suppression of TEOAE spectral constituents.

Fig.5 - Iso-suppression tuning curves of the separate frequency components of 500 m s-click evoked OAE (left) and of the overall TEOAE response (right). The tuning curves were constructed for the frequency ranges indicated above the TEOAE spectrum (bottom records). Dashed lines indicate noise level.
6. Ipsilateral Forward Masking of TEOAE
Neurons of the medial olivocochlear system (MOCS) can be effectively activated with both contralateral and ipsilateral sound (Liberman & Brown, 1986). Direct electrical stimulation of the crossed olivocochlear bundle (the subsystem of the MOCS presumably consisting of the ipsilaterally activating efferent fibers (Warren & Liberman, 1989) at the floor of the IV ventricle) has resulted in bilateral desensitization of the cochleas (Rajan, 1988, 1990), as well as in the suppression of the DPOAEs (Mountain, 1980). Therefore, it is reasonable to suggest some functional significance of the ipsilaterally activated olivocochlear feedback. Nevertheless, the latter reflex arc has received little attention in the literature. Evidence was presented for the involvement of efferent system in the ipsilateral forward-masking of the compound-action potential (Bonfils & Puel, 1987), and in the ipsilateral forward masking of TEOAE, demonstrated by the comparison between ipsilateral, contralateral, and binaural forward masking of TEOAE (Berlin et al., 1995). Nevertheless, the majority of the data related to the cochlear efferent physiology were obtained in experiments on the anesthetized animals, which may have changed reflex properties of the efferent neurons (Liberman & Brown, 1986). As a result, in an awake human being even the question about the latency of the contralateral activation of MOCS is still disputable (Lind, 1994). There are indications for the existence of the ipsilaterally activated efferent suppression of TEOAE in normal hearing subjects (Tavartkiladze et al., 1996). Comparison of the ipsilateral and contralateral efferent-mediated TEOAE suppressions in the same subject could be useful for the clinical testing of MOCS functioning.
7. Forward-Masking Experiment
As in the simultaneous-masking studies, subjects investigated were normal-hearing subjects with no history of otologic disease, with audiometric thresholds less than 20 dB within the frequency range of 125-8000 Hz, and type-A tympanograms. Absence of the spontaneous OAEs was proved by the ILO 88 analyzer (Otodynamics). Suppression of TEOAE by the continuous contralateral-noise stimulation is known to be of the same order of magnitude as the TEOAE spontaneous changes (Berlin et al., 1993) and slightly more than the TEOAE changes with directed attention (Froehlich et al., 1993). The TEOAE suppression by relatively short acoustic stimuli (clicks or broad-band noise no more than 30 ms duration) was investigated. This effect was expected to be somewhat smaller than the suppression associated with continuous noise presented contralaterally. To minimize baseline changes all TEOAE recordings were performed in one lengthy recording session without change of the probe’s position in the test ear.
The ILO88 system (with software version 3.94L) was set up with uniform (80-microseconds) linear clicks as stimulus 1 and quad-spaced clicks as stimulus 2. Stimulus 1 was routed to channel A as the ipsilateral acoustic stimulus. Stimulus 2 was fed to channel B. In click-to-click forward masking experiments, the latter stimulus was delivered ipsilaterally through the second electroacoustic transducer of ILO probe and was used as a masker. In noise-to-click forward masking experiments, output of the channel B triggered the general-purpose digital generator (HP33120A, Hewlett Packard). After appropriate attenuation (output attenuator of the Midimate 602 audiometer, Madsen Electronics) digitally generated broadband (bandwidth: 50-8000 Hz) noise bursts of 10 or 30 milliseconds duration were delivered ipsilaterally to the second electroacoustic transducer of the ILO probe or contralaterally to the TDH-39 headphone. Canceling of masking-signal artifact was observed in the course of averaging because the ILO system alternates the phase of every accepted stimulus and response. Before the execution of forward masking experiments, the TEOAE was also masked by continuous broad-band noise delivered contralaterally. In all forward masking experiments test clicks were delivered in sequence (inter-click interval 30 milliseconds) with the repetition rate of 3 Hz (Fig. 6). Such a low stimulation rate was used in order to guarantee a 200 milliseconds pause between the test series. This time was probably essential for the excitation decay in the olivocochlear reflex arc (Giraud et al., 1997; Warren & Liberman, 1989). Emission to each test click was stored separately. At least 1000 TEOAE responses were recorded without windowing. After averaging TEOAE responses in each software buffer (to different test clicks) were time-windowed (4-15 milliseconds) and their RMS amplitudes were calculated using ILO88 software. To trace baseline variations in response amplitude (see for example: (Berlin et al., 1993), TEOAE records were obtained alternatively: with and without contralateral or ipsilateral masking stimulation. Each TEOAE record in the presence of noise preceded the record without a masker. The mean differences (and their standard errors) of TEOAE amplitudes from these pairs (5 pairs per each point) were calculated. The time delay (D t) between the the onset of masker and the second test click in sequence was adjusted to be 1 to 30 milliseconds (Fig. 1). In noise-to-click forward masking experiments it was fixed at two predetermined values: 0 milliseconds (test A) or 15 milliseconds (test B). A tone-tailed test (t-test) was applied to determine the statistical significance of the TEOAE amplitude changes under masking conditions.

Fig. 6. Time patterns used in forward masking studies. For the picture clarity only the case of noise-to-click masking was illustrated. Test clicks were separated by the 30 ms interval. Test sequences were delivered with the rate of 3 Hz. Time delay (D t) between the masker onset and the second click in the test sequence varied from 1 to 30 milliseconds in click-to-click masking experiments and was equal to 0 (test A) or 15 milliseconds (test B) in noise-to-click masking experiments.
8. Contralateral Masking With Continuous Broad-Band Noise
Contralateral noise remarkably reduced the TEOAE amplitude. In one subject OAE evoked by the clicks of moderate intensity of 65.5 dB SPL (relating to subjective threshold it was 30 dB SL) were diminished by as much as 2.3 dB with the use of 60 dB SL contralateral noise. The effect was observed at the masker intensities up to 30 dB SL .
9. Ipsilateral Click-to-Click Forward Masking
TEOAEs were dramatically suppressed during the first ms after masking click delivery (Fig. 7). This suppression consisted in the overall decrease of emission-time components with maybe a more prominent reduction of long-latency emission. the amplitude of emission recovered to 90% of the control value in 5 milliseconds. Changes of emission amplitude at longer interstimulus intervals were not significant.
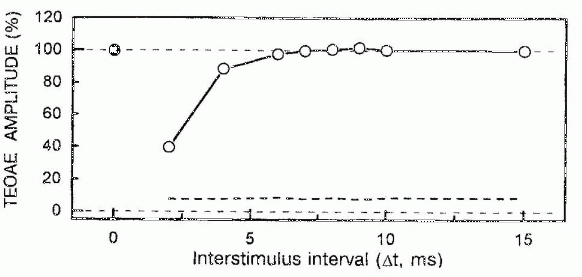
Fig.7. Changes of TEOAE amplitude with interstimulus time interval (D t) under click-to-click forward masking conditions. Filled circle indicates the control TEOAE amplitude (TEOAE amplitude to the first click in test sequence). Dashed line indicates noise level. Test click intensity = 20 dB SL; masking click intensity = 46 dB SL.
10. Contralateral Versus Ipsilateral Noise-To-Click Forward Masking
Contralateral broad-band noise burst stimulation with the intensity of 50 dB SL (65 dB nHL) evoked statistically significant reduction of TEOAE amplitude 15 milliseconds after masker onset (Fig.8). This effect became more prominent with the increase of noise duration up to 30 milliseconds. The same noise stimulation presented ipsilaterally, evoked similar effect at the intervals between masker and the test of more than 30 milliseconds (Fig. 8). These effects could not be related to the efferent feedback activation by the test sequence because there were no statistically significant changes in the amplitude of OAE evoked by different clicks in test sequence without masking (Fig. 8). The time course of ipsilateral and contralateral suppressions was practically identical. Nevertheless, the prominent ipsilateral suppression at the masker-stimulus onset delay of 15 milliseconds was also found. It corresponded to the 5 milliseconds difference between masker end and the stimulus delivery, and obviously related to the dramatic TEOAE suppression revealed in click-to-click forward masking experiment (Fig. 7).
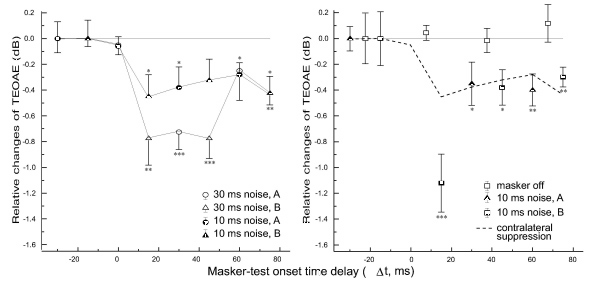
Fig 8. Temporal dynamics of contralateral (A) and ipsilateral (B) suppression of TEOAE. Each point represents the mean difference of TEOAE amplitudes (5 pairs) recorded in masker-on and masker-off conditions. Open squares on B show the mean TEOAE amplitudes without masker. The latter amplitudes were related to the mean amplitude of TEOAE evoked by the first click in test sequence. Error bars indicate standard errors.
11. Middle-Ear Reflexes
In noise-to-click forward-masking experiments noise intensities as high as 50 dB SL were used. With the noise presented continuously, this value was found to be only slightly below the threshold of stapedial muscle reflex in this subject. Nevertheless, the middle-ear reflex threshold using short-noise bursts of the maximum for our experiments duration (30 milliseconds), was determined to be as much as 80 dB SL, which is 30 dB higher than the intensity used in masking experiments . To eliminate the possibility of the middle-ear reflexes which cannot be recorded with conventional impedance measurements, the sound pressure in the outer-ear canal during alternative presentation of contralateral noise was evaluated. There was no pressure excursion at the 40 to 110 milliseconds poststimulus time when the stapedial reflex could be observed (Fisch & Schulthess, 1963; Metz, 1951). The observation could not rule out the theoretical possibility of slowly developing reflex tension in the middle-ear muscles. In order to exclude this possibility, the TEOAE amplitudes were compared without masker and just before masker onset (it is a response to the first click in test sequence). No statistically significant differences were found (Tavartkiladze et al., 1997).
12. Efferent-Mediated Effects in Ipsilateral-TEOAE Suppression
The magnitudes of the TEOAE suppression caused by contralateral continuous noise (e.g., 0.6 dB with the contralateral noise at 30 dB SL in one subject were only slightly less than previously reported mean values for normal-hearing subjects (0.7 dB with the masker intensity of 20 dB SL) (Collet et al., 1990). Hence, the excitability of the olivocochlear reflex arc (at least from contralateral side) was unlikely to differ significantly from the typical one. The dramatic decrease of the TEOAE under click-to-click forward-masking conditions at the interstimulus intervals smaller than 5 ms (Fig. 7) could be attributed exclusively to the intracochlear processes due to the longer minimal latency of the medial olivo-cochlear neuron responses to the external sound. This latency was found to be at least 6-8 ms in cat (Liberman & Brown, 1986) and 7 milliseconds in guinea pig (Brown, 1989), and there is no reason to expect the significant reduction of this time in human beings. Moreover, practically complete TEOAE inhibition observed withing the first 5 ms poststimulus (Fig. 7) correlated well with the degree of the TEOAE suppression that was reported previously for the ipsilateral simultaneous TEOAE masking (Tavartkiladze et al., 1994). Hence, the TEOAE suppression observed 5 ms after the end of ipsilateral masking noise burst (Fig. 8) may have been mainly of cochlear origin.
It is known that short acoustic clicks with relatively low repetition rate are quite ineffective in eliciting efferent response (Liberman & Brown, 1986). Longer stimuli (i.e., tone bursts of 50 milliseconds) are capable of exciting the MOCS neurons (Brown, 1989). Accordingly, noise bursts presented contralaterally or ipsilaterally elicited a statistically significant TEOAE reduction 30 milliseconds and more after the end of noise stimulation (Fig. 8). The latency of this effect from the contralateral side was found to be less than 15 milliseconds (Fig. 8). This value differs somewhat from the previously reported estimate of the contralateral TEOAE-suppression latency (less than 40-140 milliseconds) (Lind, 1994) and from the latency of the efferent-mediated ipsilateral suppression of the compound-action potential in guinea pigs [30-40 milliseconds (Bonfils & Puel, 1987]. So far no systematic studies of this question have been performed. Moreover, at the 15 milliseconds interval between contralateral noise and test click the possibility that additional TEOAE suppression related to the intracochlear processes on the ipsilateral side cannot be exclude. The degree of acoustic isolation between the two ears was not greater than 40 dB, and attenuated noise could stimulate ipsilateral cochlea owing to crosstalk. Nevertheless, the effect at longer noise-to-click intervals appears to result from MOCS activation because (1) its duration was not explained by intracochlear suppression (Fig. 7 and 8) and (2) the suppression magnitude was similar from ipsilateral and contralateral sides. The long duration of the efferent-mediated inhibition of TEOAE (more than 80 milliseconds; see Fig. 8) did not contradict previously reported TEOAE forward masking data (Gobsch et al., 1992; Lind, 1994) and other studies of ipsilateral effects (Berlin et al., 1995).
The most striking result of our experiments was the close resemblance of the magnitudes and time courses of the efferent-mediated effects elicited from contralateral and ipsilateral sides (Fig. 8). Berlin, et al. (1995) compared forward masking of TEOAE by ipsilateral, contralateral and binaural noise. In full accordance with the data presented in this chapter, they found TEOAE suppression of approximately the same magnitude (about 0.5 dB) for both ipsilateral and contralateral noise stimulation. Binaural noise stimulation caused more prominent reduction of TEOAE amplitude (about 1-1.5 dB). Differences between ipsilateral and contralateral effects were not significant 20 to 100 milliseconds after noise stimulation (Berlin et al, 1995). These results appear to contradict the results of Liberman and Brown (1986) who reported that 59% of MOCS neurons are most sensitive to the ipsilateral stimuli, 29%, to the contralateral ones, and 11%, from both. Consequently one could expect some difference in the magnitude of the efferent-mediated TEOAE suppression evoked by contralateral and ipsilateral stimulation. This discrepancy may be related to the fact that the MOCS neuron excitability pattern depends on the level of general anesthesia. Indeed, it was speculated that percent of binaurally responding MOCS neurons can be higher in less anesthetized animals (Liberman & Brown, 1986). Moreover, in unanesthetized decerebrated cats, 60% of efferent units were reported to respond to both contralateral acoustic stimulation and ipsilateral electrical stimulation (Fex, 1962, 1965). Thus, in awake human beings, a significant portion of MOCS neurons can be expected to be bilaterally activated. This could explain the similar magnitudes of the efferent-mediated TEOAE suppressions evoked by contralateral and ipsilateral stimuli.
13. References
Aran J-M: Current perspectives on inner ear toxicity. Otolaryngol Head Neck Surg 1995; 112: 133-144.
Avan P, Bonfils P, Loth D et al.: Quantitative assessment of human cochlear function by evoked otoacoustic emissions. Hear Res 1991; 52: 99-112.
Berlin CI, Hood LJ, Cecola RP et al.: Does type I afferent neuron dysfunction reveal itself through lack of efferent suppression? Hear Res 1993; 65: 40-50.
Berlin CI, Hood LJ, Wen H, Szabo P et al.: Contralateral suppression of non-linear click-evoked otoacoustic emissions. Hear Res 1993; 71: 1-11.
Berlin CI, Hood LJ, Hurley AE et al.: Binaural noise suppresses linear click-evoked otoacoustic emissions more than ipsilateral or contralateral noise. Hear Res 1995; 87: 96-103.
Bonfils P, Puel J-L: Functional properties of the crossed part of the medial olivo-cochlear bundle. Hear Res 1987; 28: 125-130.
Bray P, Kemp DT: An advanced cochlear echo suitable for infant screening. Br J Audiol 1987; 21: 191-204.
Brown M.C. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 1989; 40: 93-110.
Collet L: Use of otoacoustic emissions to explore the medial olivocochlear system in humans. Br J Audiol 1993; 27: 155-159.
Collet L, Kemp PT, Veuillet E et al.: Effect of contralateral auditory stimuli on active cochlear micromechanical properties in human subjects. Hear Res 1990; 43: 251-262.
Dallos P :Response characteristics of mammalian cochlear hair cells. J Neurosci 1985; 5: 1591-1608.
Durrant JD: Contralateral suppression of otoacoustic emissios: delay of effect? J Commun Disord 1998; 31: 485-488.
Elberling C, Parbo J, Johnsen NJ et al.: Evoked acoustic emissions: clinical application. Acta Oto-Laryngol (Stockh) 1985; Suppl 421: 77-85.
Fex J: Auditory activity in centrifugal and centripetal cochlear fibers in cat. Acta Physiol Scand 1962; Suppl 189: 2-68.
Fex J: Auditory activity in uncrossed centrifugal cochlear fibers in cat. Acta Physiol Scand 1965; 64: 43-57.
Fisch U, Schulthess G: Electromyographic studies on the human stapedial muscle. Acta Otolaryngol (Stockh) 1963; 56: 287-297.
Froehlich P, Collet L, Morgon A: Transient evoked otoacoustic emission amplitudes change with changes of directed attention. Physiol Behav 1993; 53: 679-682.
Frolenkov GI, Artamasov SV, Kruglov AV et al.: Time and frequency components of transient evoked otoacoustic emission: the input/output functions. Abstr. Eighteenth Midwinter Meet. Assoc. Res. Otolaryngol. 1995: 121.
Giraud AL, Collet L, Chery-Croze S: Suppression of otoacoustic emission is unchanged after several minutes of contralateral acoustic stimulation. Hear Res 1997; 109: 78-82.
Gobsch H, Kevanishvili Z, Gamgebeli Z et al.: Behaviour of delayed evoked otoacoustic emission under forward masking paradigm. Scand Audiol 1992; 21: 143-148.
Grandori F: Non-linear phenomena in click- and tone-burst- evoked otoacoustic emissions. Audiol 1985; 24: 71-80.
Grandori F, Ravazzani P: Non-linearities of click-evoked otoacoustic emissions and the derived non-linear technique. Br J Audiol 1993; 27: 97-102.
Hauser R, Probst R, Lohle E: Click- and tone-burst-evoked otoacoustic emissions in normally hearing ears and in ears with high-frequency sensorineural hearing loss. European Arch Oto-Rhino-Laryngol 1991; 248: 345-352.
Hilger AW, Furness DN, Wilson JP: The possible relationship between transient evoked otoacoustic emission and organ of Corti irreguliarities in the guinea pig. Hear Res 1995; 84: 1-11.
Hill JC, Prasher DK, Luxon LM: Latency of contralateral sound-evoked auditory efferent suppression of otoacoustic emissions. Acta Otolaryngol (Stockh) 1997; 117: 343-351.
Kemp DT: Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am 1978; 64: 1368-1391.
Kemp DT: Towards a model for the origin of cochlear echoes. Hear Res 1980; 2: 533-548.
Kemp DT: Otoacoustic emissions, traveling waves and cochlear mechanisms. Hear Res 1986; 22: 95-104.
Kemp DT, Chum RA: Properties of the generator of stimulated acoustic emissions. Hear Res 1980; 2: 213-232.
Kemp DT, Bray P, Alexander L et al.: Acoustic emission cochleography: practical aspects. In Cianfrone G, Grandori F (eds): Cochlear Mechanics and Otoacoustic Emissions: Scand Audiol 1986; Suppl. 25: 71-95.
Kossl M, Russell IJ: The phase and magnitude of hair cell receptor potentials and frequency tuning in guinea pig cochlea. J Neurosci 1992; 12: 1575-1586.
Kubo T, Sakashita T, Hachikawa K et al.: Frequency analysis of evoked otoacoustic emissions. Acta Oto-Laryngol 1991; Suppl. 486: 73-77.
Liberman MC, Brown MC: Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 1986; 24: 17-36.
Lind O: Contralateral suppression of TEOAE. Attempts to find a latency. Br J Audiol 1994; 28: 219-225.
Lonsbury-Martin BL, Martin GK, Probst R et al.: Spontaneous otoacoustic emissions in nonhuman primate. II. Cochlear anatomy. Hear Res 1988; 33: 69-94.
Martin GK, Lonsbury-Martin BL, Probst R et al.: Spontaneous otoacoustic emissions in a nonhuman primate. I. Basic features and relations to other emissions. Hear Res 1988; 33: 49-68.
Norton SJ, Neely ST: Tone-burst-evoked otoacoustic emissions from normal-hearing subjects. J Acoust Soc Amer 1987; 81: 1860-1872.
Metz O: Studies on the contraction of the tympanic muscles as indicated by changes in the impedance of the ear. Acta Otolaryngol (Stockh) 1951; 39: 397-405.
Mountain DC: Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science 1980; 210: 71-72.
Probst R, Coats AC, Martin GK et al.: Spontaneous, click-, and toneburst-evoked otoacoustic emissions from normal ears. Hear Res 1986; 21: 261-275.
Probst R: Otoacoustic emissions: An overview. In Pfaltz CR (ed): New aspects of cochlear mechanics and inner ear pathophysiology. Adv Otorhinolaryngol, 44: pp 1-91. Karger, Basel, 1990.
Probst R: A review of otoacoustic emissions. J Acoust Soc Am 1991; 89: 2027-2067.
Rajan R: Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity. I Dependence on electrical stimulation parameters. J Neurophysiol 1988; 60: 549-567.
Rajan R: Functions of the efferent pathways to the mammalian cochlea. In Rowe M, Aitkin L (eds): Information processing in mammalian auditory and tactile systems, pp 81-96. Wiley Liss, New York, 1990.
Tavartkiladze GA, Frolenkov GI, Kruglov AV: On the site of the evoked otoacoustic emission generation. In Proc. XII Biennal Symposium of the International Electric Respons audiometry Study Group, (Terme Di Comano (TN) - Italy, September 25-29, 1991), 1991: 58.
Tavartkiladze GA, Frolenkov GI, Kruglov AV: Delayed evoked otoacoustic emission and mechanisms of its generation. Sensory Systems 1993; 7: 85-99.
Tavartkiladze GA, Frolenkov GI, Kruglov AV et al.: Ipsilateral suppression effects on transient evoked otoacoustic emission. Br J Audiol 1994; 28: 193-204.
Tavartkiladze GA, Frolenkov GI, Artamasov SV: Ipsilateral suppression of transient evoked otoacoustic emission: role of the medial olivocochlear system. Acta Otolaryngol (Stockh) 1996; 116: 213-218.
Tavartkiladze GA, Frolenkov GI, Kruglov et al.: Ipsilateral suppression of transient evoked otoacoustic emissions. In Robinette MS, Glattke TJ (eds): Otoacoustic Emissions: Clinical Applications, chapt.6, pp 110-129. Thieme, New-York-Stuttgart, 1997.
Veuillet E, Collet L, Duclaux R: Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: Dependence on stimulus variables. J Neurophysiol 1991; 65: 724-735.
Veuillet E, Collet L, Morgon A: Differential effects of ear-canal pressure and contralateral acoustic stimulation on evoked otoacoustic emissions in humans. Hear Res 1992; 61: 47-55.
Warren EH, Liberman MC: Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear Res 1989; 37: 89-104.
Wilson JP: Evidence for a cochlear origin for acoustic re-emissions, threshold fine-structure and tonal tinnitus. Hear Res 1980; 2: 233-252.
Wilson JP, Sutton GJ: Acoustic correlates of tonal tinnitus. In Tinnitus: Ciba Foundation symposium, pp 82-107. Pitman Books, London: 1981.
Zurek PM: Spontaneous narrowband acoustic signals emitted by human ears. J Acoust Soc Amer 1981; 69: 514-523.
Zurek PM: Acoustic emissions from the ear: A summary of results from humans and animals. J Acoust Soc Am 1985; 78: 340-344.






